
Role of Real World Evidence in Pharma (RWE in Pharma)
Shots:
-
Real-world evidence (RWE) aims to mitigate the limitations of randomized controlled trials (RCTs) by keeping a constant vigil on the safety and effectiveness of drugs in real-world settings
-
Phrases such as Real-world data, Randomized Controlled Trials, and Real-World Evidence hold a degree of paramount importance when it comes to safeguarding the health of individuals per se
-
The article navigates the bright aspects of RWE, sheds light on the regulatory framework of RWE, discusses the 21st Century Cures Act, and provides a brief account of leading companies that provide RWE Solutions
With innovative therapies, pharma companies ferociously compete with one another to assert their dominance in the market. In the race for supremacy, companies leave no stone unturned. To emerge victorious, pharma companies leverage the use of flexible manufacturing with CDMOs, intelligent automation with AI and ML, robotic process automation (RPA) to save time in menial work, and the list goes on and on. Drugs tested on homogenous patient groups in randomized controlled trials (RCTs) may not always be evident in their safety and effectiveness in real-world settings.
RWE provides a comprehensive study on the effectiveness of the approved drugs in real-world settings. In this article, we will navigate the importance of real-world evidence in several aspects of drug development and dive deep into what the future of the pharmaceutical industry looks like with real-world evidence.
Breakdown on Real-World Data & Real-World Evidence
Real-world data is often confused with real-world evidence and used interchangeably, but there is a major distinction between these two phrases.
Any data gathered outside of randomized controlled trials can be termed real-world data. Be it electronic health records, claims reports, patient-generated data, or electronic case report forms, all come under the domain of real-world data.
When the gathered RWD is transformed using advanced analytics it turns into RWE. Real-world evidence helps provide a broader perspective on how innovative therapies perform outside the traditional randomized controlled trials
Randomized Controlled Trials vs. Real-World Evidence
Randomized controlled trials offer deep understanding in establishing the efficacy and safety of drugs but when it comes to studying the effectiveness of approved drugs in real-world settings, real-world evidence emerges as a game changer. The table below represents the variables that are taken into account for the study in randomized controlled trials (RCTs) and real-world evidence

Real-world evidence can be leveraged in multiple aspects of a drug life cycle and helps companies cut costs by deploying a unified yet precise approach to pharma companies, especially seed-stage companies. Let’s unfold the lucrative potential that Real-World Evidence offers in various stages of a drug’s life cycle:
Preclinical: RWE can be leveraged in the study of the history and epidemiology of a disease and at the same time can be used to evaluate the unmet needs of the patients and draw conclusive suggestions from them. Moreover, RWE can be used in patient characterization and identifying target populations
Clinical Development: By analyzing real-world data from the EHR, companies can utilize the processed analytical data as RWE to identify potential patients for better outcomes in clinical studies and endpoints
Regulatory Requirements: RWE is widely used by regulatory authorities to complement evidence generated from clinical studies and even as primary evidence for drug approval. Moreover, RWE helps pharma companies achieve label expansion of approved drugs
Post Marketing: Pharma companies rely heavily upon RWE to check the safety and effectiveness of the approved drugs. RWE also enhances market access through pharmacovigilance, drug utilization, and long-term follow-up. RWE helps guide pharma companies on the off-label use of approved drugs
Regulatory Efforts to Promote RWE
Regulatory bodies are steadfastly working to integrate RWE to check the effectiveness of approved drugs. Regulatory bodies such as the FDA and EU are integrating RWE to complement the clinical trial evidence. The timeline below depicts the growing significance of RWE through the years.
Navigate the evolution of RWE in the pharmaceutical industry with this illuminating infographic.
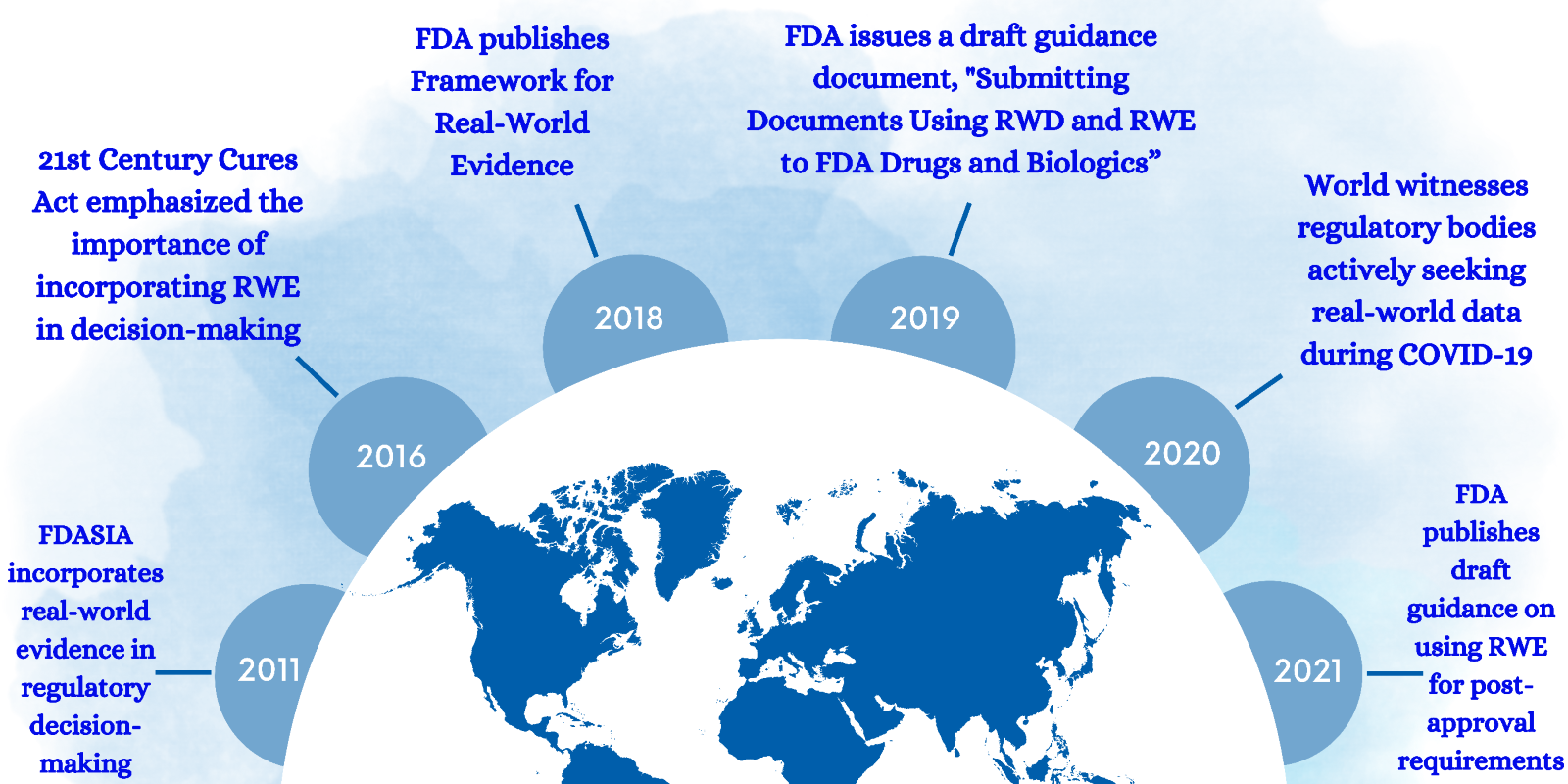
21st Century Cures Act (Cures Act)
On December 13th, 2016, the 21st Century Cures Act was signed into law, allowing the expedition of medical product development, and bringing new-age innovations to patients. It aimed to encourage pharmaceutical companies to use RWE to speed up drug development. Under this act, the FDA produced a Framework to guide pharma companies to use RWD and RWE in enhancing the effectiveness and decision-making in clinical trials.
Key Companies Involved
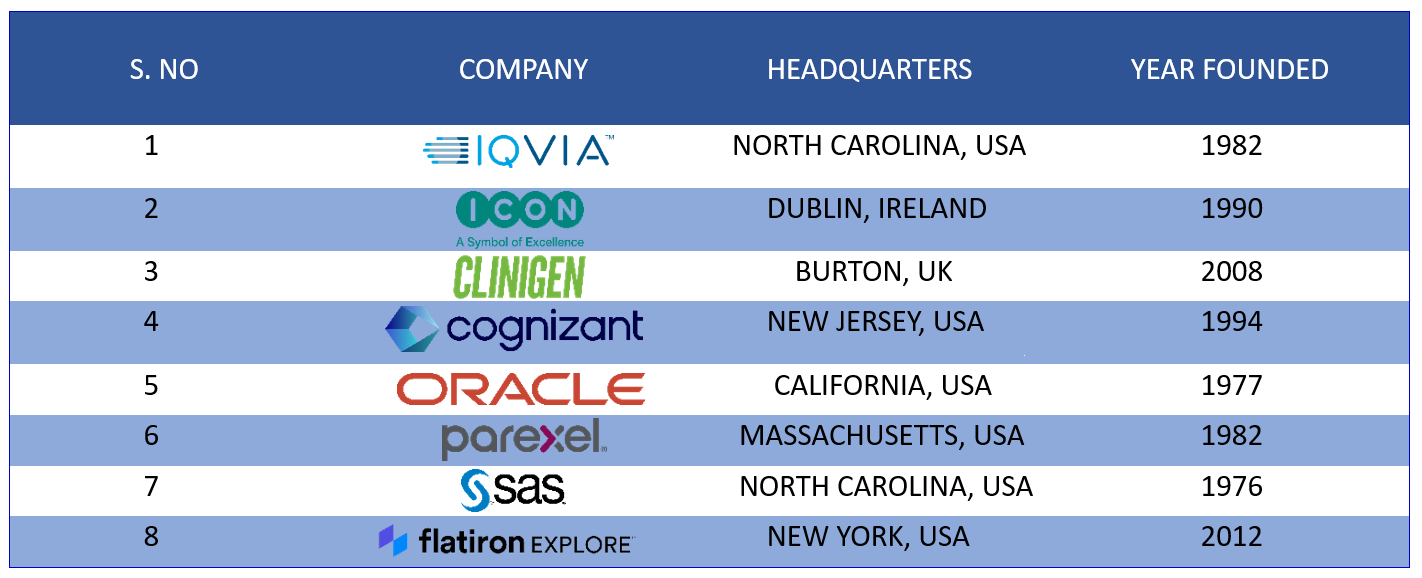
Real-world evidence Solution companies are witnessing a dramatic upthrust in terms of revenue. The global market size of the real-world evidence solution companies was predicted to be around $1.5B in 2022 and is envisioned to touch the threshold of 2.9B by 2027 with a CAGR of 15.2%. Pharma companies heavily rely on the analytics produced by real-world evidence solution companies and use the analytics to enhance the effectiveness of their products. Listed below are a few companies known for delivering real-world evidence solutions to the top pharmaceutical companies. For the sake of readability, we have kept our report crisp and concise. For the complete report, reach out to us at connect@pharmashots.com
-
IQVIA- With its advanced analytics, IQVIA emerged as a renowned name among RWE solution companies. IQVIA operates in more than 100 countries and is as a global leader in safeguarding patient privacy. As of December, 2023, IQVIA has a market cap of $39.58B
-
ICON plc- An Ireland-based CRO, ICON plc offers solutions that span the entire life cycle of drug development and commercialization. Apart from DCT solutions, ICON plc also works extensively in providing real-world intelligence with real-world data and analytics to life science companies. As of December 2023, ICON plc has a market cap of $22.18B
-
Clinigen Group plc- With services ranging from clinical trial sourcing and clinical supplies management to managed access and pharmacovigilance, Clinigen Group plc helps life science companies with pertinent data on drugs in real-world settings. A considerable share of its profit comes from European regions and the United States. The company currently has a market cap of $1.42B
-
Cognizant- An American multinational IT and consulting company, Cognizant holds a prominent place among RWE solution companies. As of December 2023, the company has a market value of $35.16B
Real World Evidence Solution by Octavus Consulting

With a global network of practitioners, patients, and key opinion leaders, PharmaShots’ parent company Octavus Consulting, is well accustomed to the trending practices in the industry and guides life science companies with its high-quality real-world data and analytics. Octavus undertakes a painstaking process to screen real-world data and further analyzes and processes relevant information with data visualization tools to prepare evidence to guide companies in their clinical studies. For more information reach out to us at bd@octavusconsulting.com
Conclusions & Perspectives
Real-world evidence comes in handy when checking the effectiveness of medications in real-world settings. It would be unwise to rely completely on the efficacy of drugs and deem them safe and effective based on RCT results. RWE, in such scenarios, can guide life science companies to make the right decision to enhance the effectiveness of drugs and make them safe for everyone. In randomized controlled trials (RCTs), several parameters like heterogeneity, geography, and demography are often not taken into account, leading to adverse drug events in many cases. To strengthen the healthcare system, it is indeed necessary to implement novel ways to check the effectiveness and safety of drugs in real-world settings.
Related Post: Robotic Process Automation in Pharmaceutical Industry (RPA in Pharma)
Tags

Saurabh is a Senior Content Writer at PharmaShots. He is a voracious reader and follows the recent trends and innovations of life science companies diligently. His work at PharmaShots involves writing articles, editing content, and proofreading drafts. He has a knack for writing content that covers the Biotech, MedTech, Pharmaceutical, and Healthcare sectors.














